Medical Device Assembly
Clean, Precise, Repeatable
Need FDA-compliant medical device assembly equipment?
AAT designs and manufactures 21 CFR Part 11 compliant medical device assembly systems with sterile component handling, precision assembly capabilities within ±0.0005 inches, and complete data logging for regulatory compliance. Our systems handle catheter assembly, syringe manufacturing, surgical instrument production, and diagnostic device assembly.
What medical device assembly processes can be automated?
We automate pin insertion for surgical devices, ultrasonic welding for disposable medical components, corona treatment for dental instruments, vision-guided quality inspection, sterile packaging integration, and multi-component assembly with automated reject handling. Typical cycle times range from 2-45 seconds per device depending on complexity.
Do you meet medical device manufacturing regulations?
Yes. Our medical automation systems comply with FDA regulations, 21 CFR Part 11 for electronic records, cGMP requirements, ISO 13485 quality standards, and are designed for clean room environments up to Class 10,000 specifications.
Start a Project With Us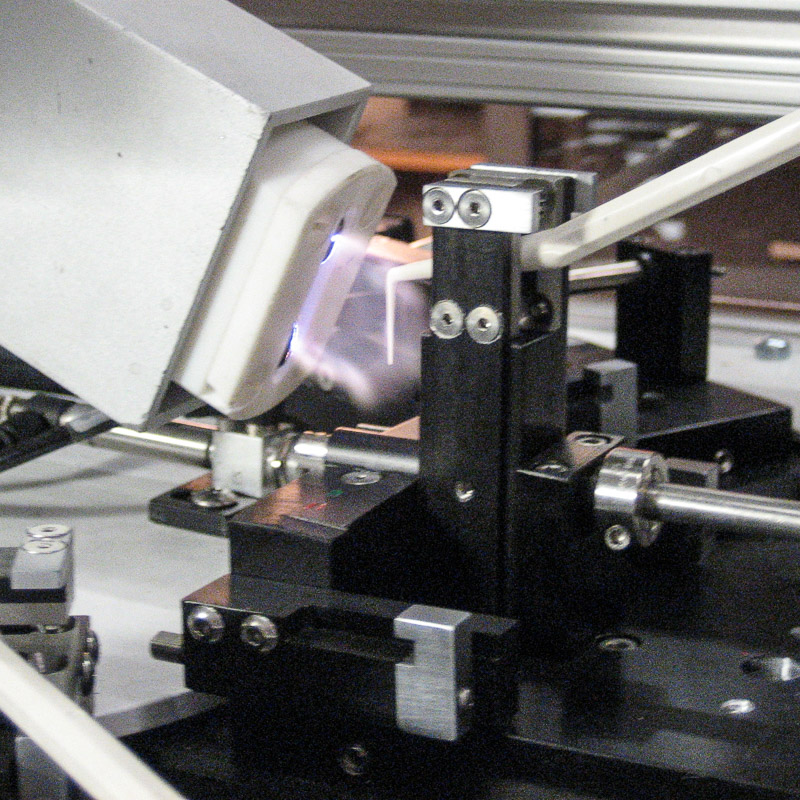
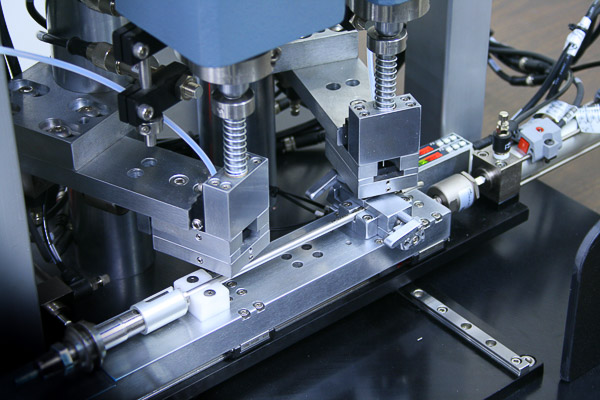
Surgical Device Pin Inserter
Pins are loaded from vibratory feeder bowls down tubes and into an escapement. An operator places the surgical device under the insertion heads. The operator activates the dual-palms. The pins are escaped and pressed into the device.

Dental Printing System
Dental tools are loaded by hand onto nests on a carre. The parts index to a corona treatment station where they are raised 90 degrees and hit with corona discharge plasma. The parts cycle to the print stations where they receive two color bands on each side, creating a continuous band in which the two sides cannot be distinguished by eye. The parts are offloaded to a curing oven.
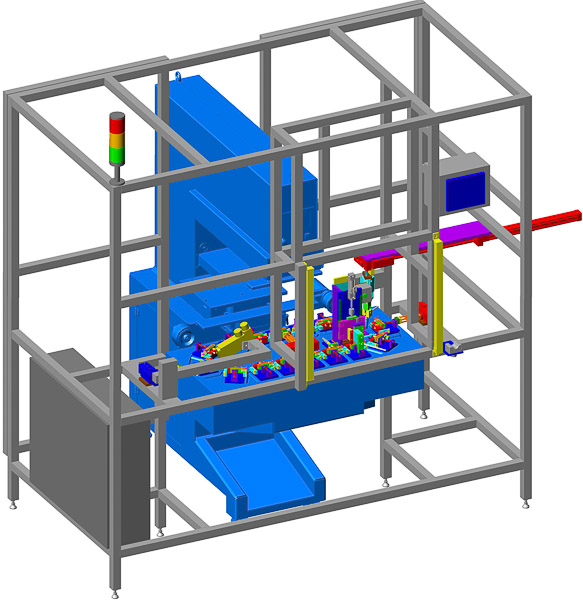
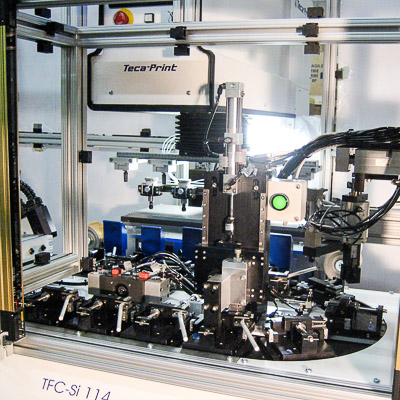
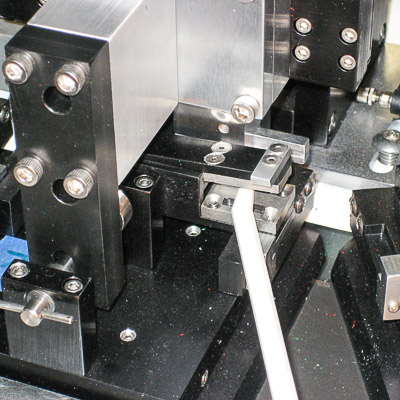
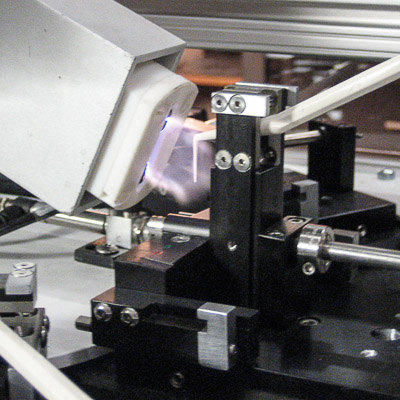
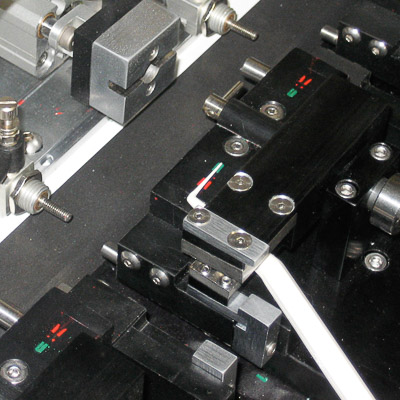
Medical Switch Assembly System
Bottom parts are loaded by vibratory feeder bowl down vibratory tracks and into custom indexing conveyors. Parts are picked up and loaded 8-up onto a rotary dial. Top parts are loaded in a similar manner and assembled onto the bottom parts. The parts are ultrasonically welded and printed with an indicator. A vision inspection system qualifies proper assembly and printed image. Good parts are offloaded to an eject bin and failed parts are offloaded to a reject bin.

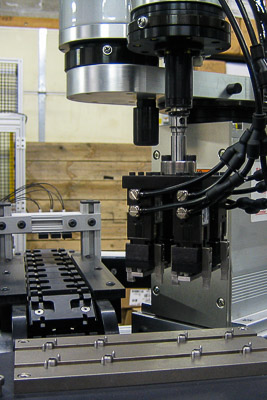

Catheter Assembly Systems
Automated tip attachment, lumen insertion, balloon mounting, and marker band placement with vision-guided quality verification and sterile packaging integration. Handles multiple catheter types with quick changeover capabilities.
Syringe Manufacturing Equipment
High-speed syringe assembly with needle attachment, plunger insertion, safety cap application, and automated inspection. Includes dose accuracy verification and packaging automation for pharmaceutical applications.
Precision Capabilities
- Positioning Accuracy: ±0.0005 inches for critical component placement
- Repeatability: ±0.0002 inches across production runs
- Force Control: 1 ounce to 500 pounds with real-time monitoring
- Cycle Times: 2-45 seconds per device (depending on complexity)
Regulatory Compliance Features
- 21 CFR Part 11: Electronic signature and audit trail capabilities
- Data Logging: Complete process parameter recording and storage
- Clean Room Compatibility: Class 10,000 to Class 100,000 environments
- Material Traceability: Lot tracking and batch record integration
- Validation Support: IQ/OQ/PQ documentation and testing protocols

